
Where E is the energy gained, q is the charge of the.

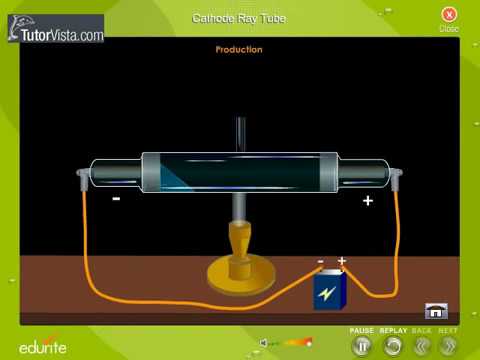
Note the above argument is for a Gas Filled Discharge Tube. The energy gained by the electrons in a cathode ray tube can be calculated using the formula: E qV. For example, if you want more bluish light, you would want to apply a higher voltage than if you want more reddish light (photons corresponding to blue light have higher energy than those corresponding to red light). This experimental test can also be easily carried out with our cathode tube, as it is provided with a pair of deflection plates positioned close to the beam. The energy to which molecules are excited to depend on the electric field through the gas and therefore you can control the wavelength of the emitted light by adjusting the voltage applied across the tube. The energy of the photons (and therefore colour of light that we see) depends on the energy of the state in which the excited molecule relative to the energy it has after it is de-excited. When the excited electrons go back to a lower energy state, they emit photons in the process. Measuring separately the electric charge ((e)) and the rest mass ((m)) of an electron is a difficult task because both quantities are extremely small ((e) 1. Thomson found that this value comes to \( 1.76 \times 10^8 C/g \).The high voltage causes the gas molecules in the tube to get excited or ionised if the electric field is strong enough. The charge-to-mass ratio of the electron can be determined by measuring the effects of the magnetic and electric fields on the motion of the beam.What is the charge-to-mass ratio for the cathode ray particles?.If the correct combination is reached, the can completely cancel each other out and not deflect the rays at all. When both electric and magnetic forces are applied to the cathode ray, they combine against or with each other to deflect the cathode rays.What is the effect of simultaneous electric and magnetic fields on the cathode ray?.Again, this is due to the negative charge the cathode rays possess. When the north pole is facing towards the CTR, the beam is deflected upwards. The electron gun will emit electrons with a kinetic energy equal to the charge of an electron times the accelerating voltage, V acc. Figure 1: A cross-section of the electron gun inside the cathode ray tube. If the south pole is facing towards the CTR (Cathode Ray Tube), the beam is deflected down. Enclosed within the cathode ray tube is an electron gun, Figure 1, which will be used to produce electrons with given energy. When a magnetic field is applied, the cathode rays get deflected.different voltages applied to the two deflecting plates of the cathode ray tube. What is the effect of a magnetic field on the cathode ray? What are the objectives of this experiment.This is because the cathode rays are negatively charged. When an electric field is applied to a stream of cathode rays, they are deflected towards the positive plate.What is the effect of an electric field on the cathode ray?.Notice the dotted circle indicating the position of the coils, the direction of the magnetic field between the coils and the value of the field. In the Thomson applet, set the voltage at 0 V and move the slider for the current to the right. e magnitude of the charge of an electron in coulombs 1.602 x 10-19 coulombs. When a current flows through the coils, a uniform magnetic field is created in the region between the horizontal plates. m mass of an electron in kg 9.10938356 × 10-31 kilograms. Using the same tube as shown in Figure 1 (above), he placed a pair of coils outside of the tube and on either side of the horizontal plates (Figure 3, below). This is the basis of the important experiments carried out by J J Thomson and others. This cathode ray can be focused and deflected and can carry small currents. Thomson also used a magnetic field to affect the beam of cathode rays. However, if there is a small hole in the anode, some electrons will pass through, forming a beam of electrons that came from the cathode or a cathode ray. In the Thomson applet, this angle is provided by selecting Deflection Angle in the Options menu. From measurements of this deflection, he was able to calculate the angle of deflection. Table of Contents 1 Cathode ray and cathode-ray tube 2 Thomson's experiments 3 Thomson's hypotheses 4 Associated articles Cathode ray and cathode-ray tube Before directly jumping Thomson's findings, let us understand some basic knowledge on cathode rays and the cathode-ray tube. Thomson measured the deflection of the beam using a ruler etched on the end of the tube. You can see a simulation of this glow on the far right of the applet diagram, as shown in Figure 2. In Thomson’s experiment, a fluorescent material was coated on the end of the tube to produce a glowing dot where the cathode rays hit.


 0 kommentar(er)
0 kommentar(er)
